Quality Assurance System
Genetic analysis based on the pharmaceutical GLP-compliant quality assurance system (RG quality assurance standards)
We introduce and operate "RG Quality Assurance Standards" for genetic analysis studies on the Criteria for Conduct of Nonclinical Studies on the Safety of Drugs (GLP Ordinance)
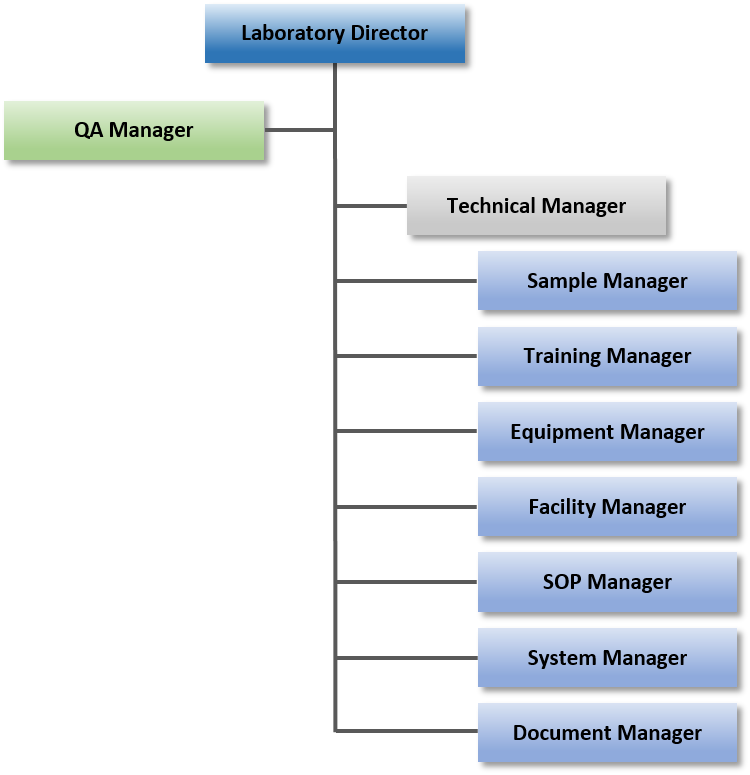
ISO15189 accreditation
RIKEN GENESIS CO., LTD. has obtained ISO15189 accreditation for genetic analysis service (accredited in December 2018).
ISO15189 is an international standard for quality management systems in clinical laboratories, accredited by Japan Accreditation Board (JAB), and is used as a standard for accuracy control in medical institutions and clinical laboratories.
